Publications
As a breakthrough technology, the MFM® has been studied and discussed in numerous publications.
The Multilayer Flow Modulator (MFM®) technology represents a paradigm shift in the treatment of aneurysms. Since 2009 (for the peripheral device) and 2011 (for the aortic device), more than 4000 MFM® devices have been implanted, demonstrating a strong record of safety and performance. Clinical investigations — some completed and published, some currently ongoing — also demonstrate the unique capabilities of the MFM® when it comes to preserving the patency of branch arteries and preventing serious complications such as spinal cord ischemia and renal failure.
 PERIPHERAL MFM®
PERIPHERAL MFM®
 AORTIC MFM®
AORTIC MFM®
The STRATO trial of the Aortic MFM® enrolled 23 patients suffering from complex Crawford type II and III thoracoabdominal aortic aneurysms (TAAA) between April 2010 and February 2011.
Three-year safety and performance results are very solid with 11 patients remaining alive and no device-related deaths. The ratio of aneurysm flow volume to total volume decreased 83%, while the ratio of thrombus volume to total volume increased 159%. There were no cases of spinal cord injury and no cases with renal or peripheral complications. Branch patency was 97%.
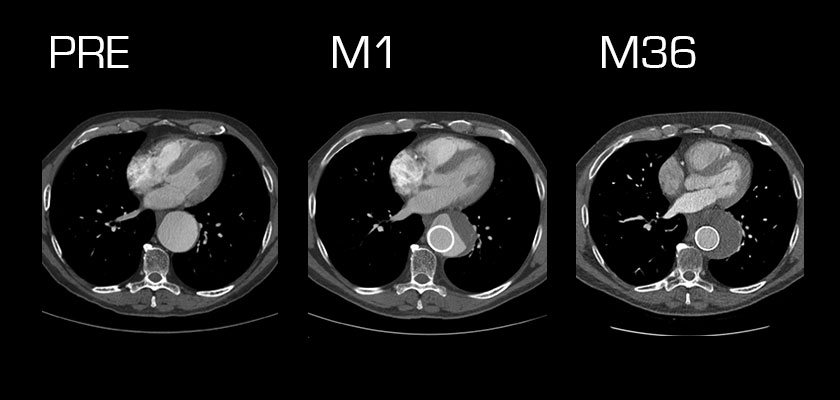
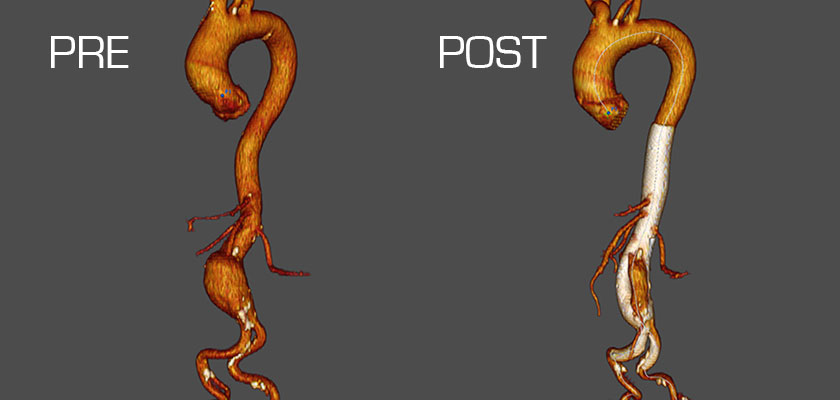
The international prospective, nonrandomized Bifurcated Aortic MFM® STREAMLINER study is currently screening and enrolling patients suffering from complex thoracoabdominal aortic aneurysms at up to 12 centers in Europe, Israel, Morocco and Turkey. As of June 2016, 23 patients had been enrolled, out of a targeted total of approximately 40.
In an interim report of results for the first patients 6 months of follow-up, technical success was 100%, with 0% mortality. At 1 month, flow into the aneurysm was decreased for 88.9% of patients. At 6 months, 100.0% of patients had decreased flow, and 10.0% had total aneurysm thrombosis. The patency of covered branch vessels was 100% at 1 and 6 months. There were no cases of device migration and no endoleaks.